

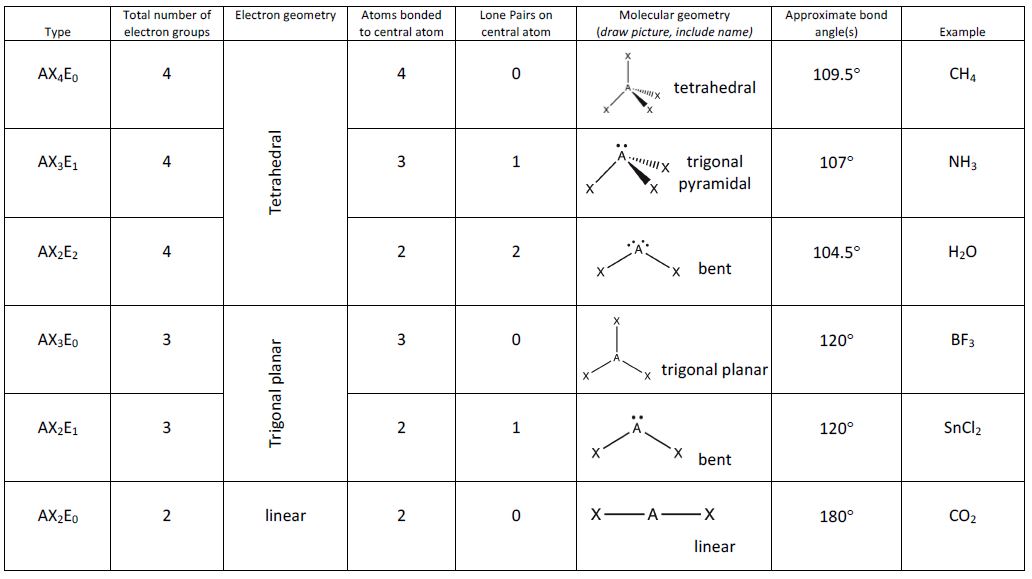
SF 6 has 6 bonding atoms and no lone pairs the structure is octahedral.48 original VEs - 48 involved in bonded atoms = 0.
Electron domain molecular geometry table full#
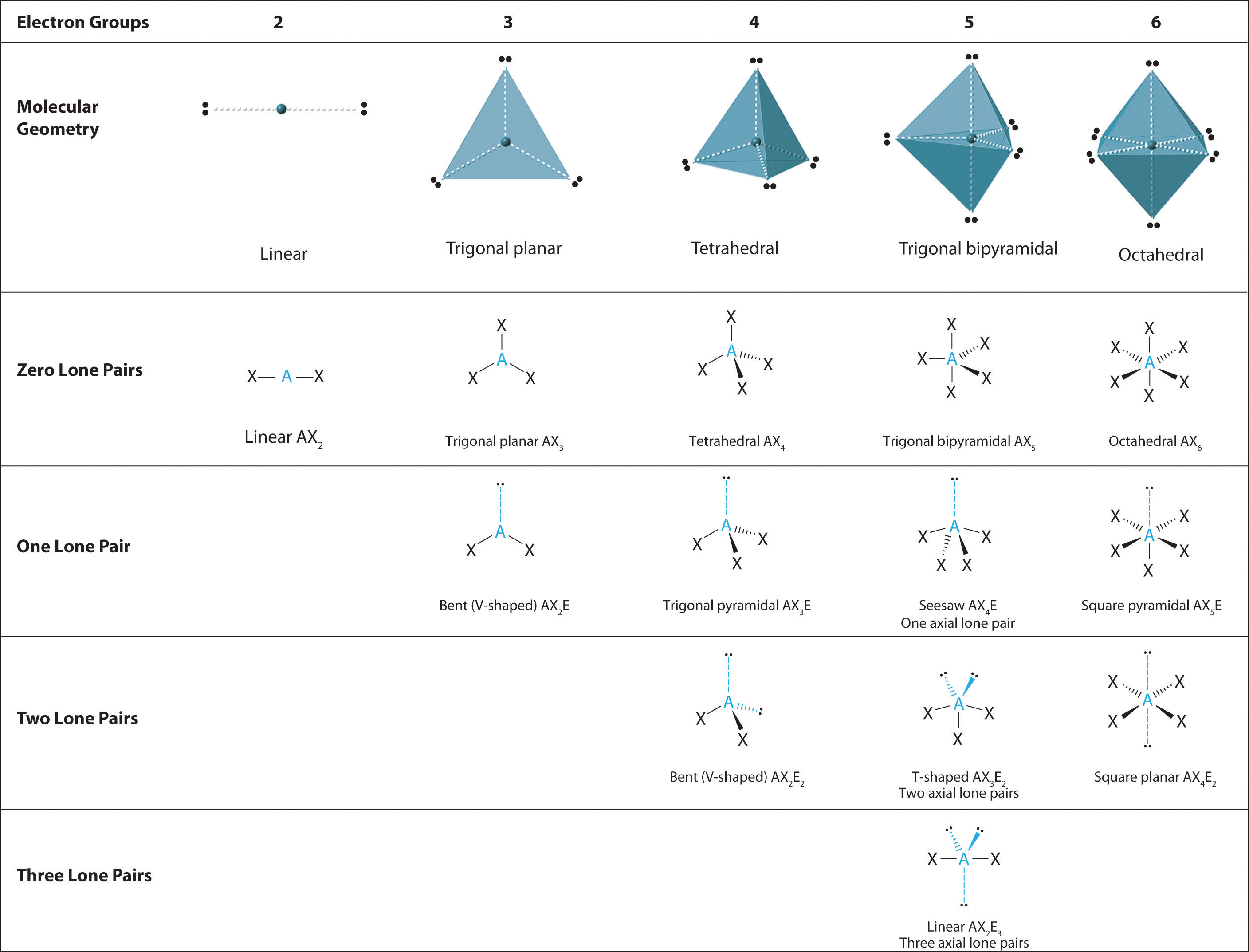
2) has 18 valence electrons andĢ bonds around the central atom which require 1: Determining # lone pairs in carbon dioxide Since there are two bonding atoms and no lone pairs around the central carbon atom, there must be multiple bonds, either two double bonds or a single and triple bond, to conform to the octet rule and the correct structure. The structure must be linear since it has two bonding atoms and no lone pairs. (16 – 16 = 0) means there are no VEs left for lone pairs on the central C atom. Two atoms bonded to the central atom (2 bonds x 8 VE/atom) means that there are 16 VEs associated with those terminal atoms. 1), which has 16 valence electrons and two bonding atoms on the central C atom. Now determine what the structure is by finding the structure in the VSEPR table that has the correct number of bonding atoms and lone pairs.Ĭonsider CO 2 (Fig. If there are remaining valence electrons, they must be lone pairs (LPs) around the central atom, so the remaining electrons are divided by two to come up with the number of lone pairs. So all one has to do is count the number of valence electrons in the structure, subtract the number of valence electrons involved in a bonded atom, eight for all bonded atoms, according to the octet rule, except for H, which requires two. Any valence electrons left over will have to be incorporated as lone pairs around the central atom.

The basic idea is that in any Lewis structure, all atoms (except hydrogen), whether single, double or triple bonded require eight valence electrons (VEs). 6 The method is primarily targeted for chemistry students who already have an understanding of Lewis structures and are focusing on molecular geometry. However, there is a quick and easy method to determine VSEPR structures based on the octet rule that does not require drawing a Lewis structure or using complicated equations. Textbooks (1-3) and online resources (4-5) teach that it is first necessary to draw a Lewis structure before determining the VSEPR shape.


 0 kommentar(er)
0 kommentar(er)
